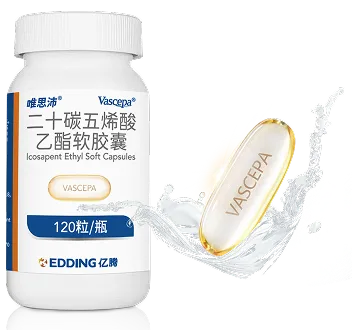
Icosapent Ethyl Soft Capsules



The following product may not have been approved and/or licensed for marketing in all countries where this website is accessible.
(for medical professionals only)
Email: pv@eddingpharm.com
Hotline: 0512-67611023
【Name】 | Generic Name:Icosapent Ethyl Soft Capsules Brand Name:VASCEPA®(唯思沛®) Chinese Pinyin:Ershitanwuxisuanyizhi Ruanjiaonang |
【Ingredients】 | Active Ingredient: icosapent ethyl Excipients:gelatin, glycerin, maltitol, sorbitol,purified water and medicinalInk. |
【Appearance】 | VASCEPA, a lipid-regulating agent, is supplied as either a 0.5 gram or a 1 gram amber-colored, liquid-filled soft gelatin capsule for oral use. |
【 Indications】 | As an adjunct to diet to reduce TG levels in adult patients with severe (≥ 500 mg/dL) hypertriglyceridemia. As an adjunct to statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥ 150 mg/dL) and established cardiovascular disease or diabetes mellitus and 2 or more additional risk factors for cardiovascular disease. The effect of VASCEPA on the risk for pancreatitis in patients with severe hypertriglyceridemia has not been determined. |
【Specification】 | 1.0g |
| For detailed product information, please download the full package insert (PDF). | |
(for medical professionals only)
Email: pv@eddingpharm.com
Hotline: 0512-67611023
Year: 2017
Year: 2023
Year: 2019