Product Overview
Guideline Recommendations

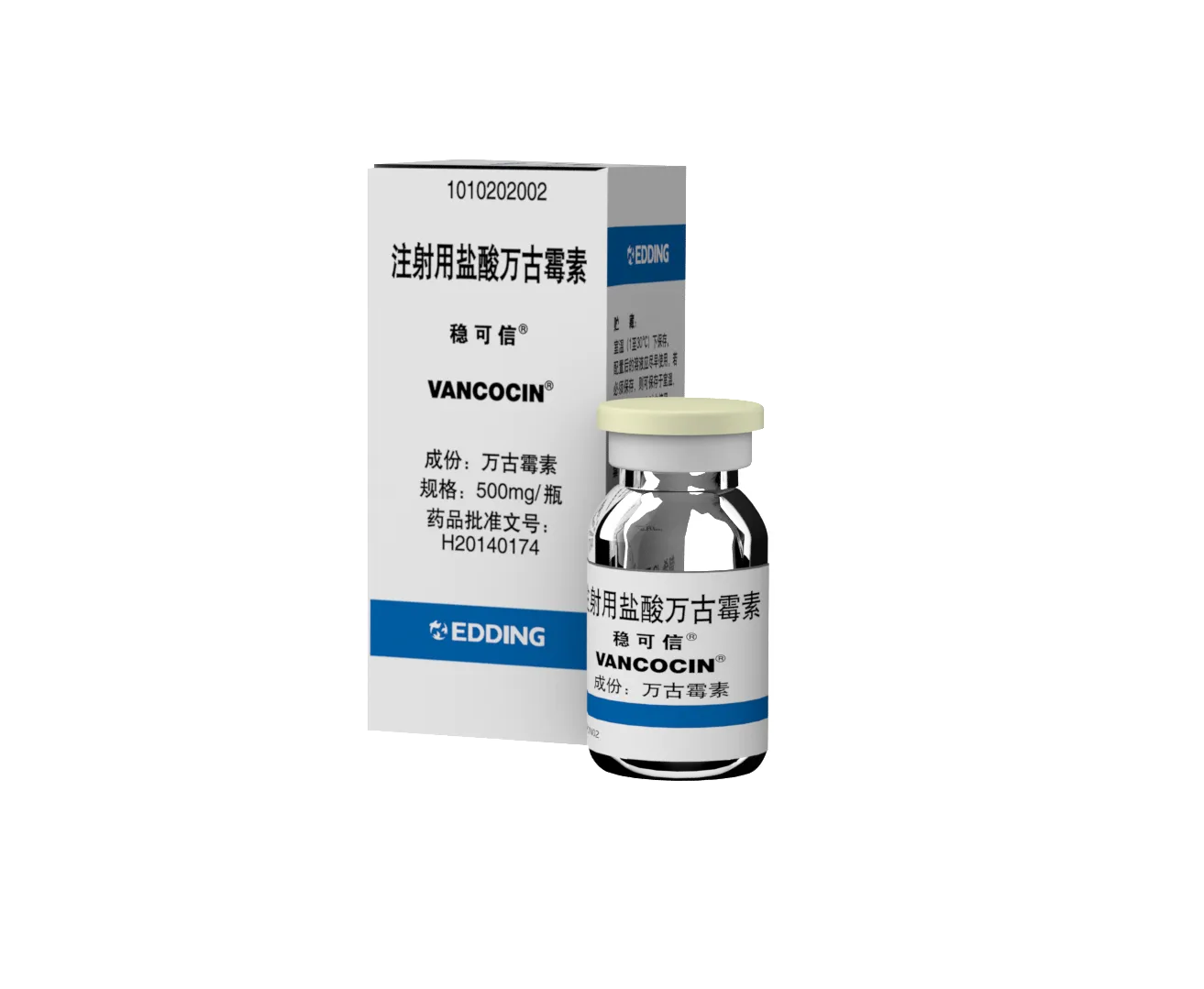
Vancomycin Hydrochloride for Injection
The most extensive indications
With eleven approved indications, vancomycin is the primary agent used to treat serious, multidrug-resistant Gram-positive infections across many body sites.
Clinically Proven Potency with a Well-Established Safety Profile
Vancomycin exerts potent and sustained bactericidal activity through a unique triple mechanism of action.
MRSA and MRCNS exhibit zero drug resistance to vancomycin,according to CHINET data, while Enterococci shows very low resistance rates.
With widely adopted Therapeutic Drug Monitoring (TDM), clinicians can effectively monitor and manage the safety of vancomycin therapy.
MRSA and MRCNS exhibit zero drug resistance to vancomycin,according to CHINET data, while Enterococci shows very low resistance rates.
With widely adopted Therapeutic Drug Monitoring (TDM), clinicians can effectively monitor and manage the safety of vancomycin therapy.

Guideline-Recommended First-Line Therapy
Numerous domestic and international guidelines and consensuses recommend vancomycin as the first-line therapy for multidrug-resistant gram-positive bacteria, especially methicillin-resistant Staphylococcus aureus (MRSA).

The following product information is intended for use by healthcare professionals only.
The following product may not have been approved and/or licensed for marketing in all countries where this website is accessible.
The following product may not have been approved and/or licensed for marketing in all countries where this website is accessible.
Directions for Use Download
(for medical professionals only)
(for medical professionals only)
Adverse Event Reporting
Email: pv@eddingpharm.com
Hotline: 0512-67611023
Product information stated by NMPA in Approved Drug Catalog of China.
【Drug Name】 | Generic Name: Vancomycin Hydrochloride for Injection Brand Name: Vancocin®(稳可信®) Chinese Pinyin: ZHU SHE YONG YAN SUAN WAN GU MEI SU |
【Ingredients】 | Active Ingredient: Vancomycin Excipients:Mannitol, hydrochloric acid, water for injection, nitrogen gas. |
【Description】 | This product is a white or off-white lyophilized block. |
【Indications】 | For infections caused by methicillin-resistant Staphylococcus aureus (MRSA) and other bacteria: Bacteremia, Infective Endocarditis, Osteomyelitis, Arthritis, Secondary superficial infections (e.g., of surgical wounds), Pneumonia, Lung abscess, Empyema, Peritonitis, Meningitis. |
【Specifications】 | 500mg |
| For detailed product information, please download the full package insert (PDF). | |
Directions for Use Download
(for medical professionals only)
(for medical professionals only)
Adverse Event Reporting
Email: pv@eddingpharm.com
Hotline: 0512-67611023
Guideline Recommendations