Product Overview
Guideline Recommendations
Academic Publications

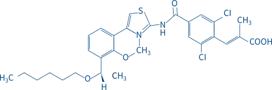
Lusutrombopag Tablets
Original imported quality
The only platelet-boosting drug with child-resistant packaging, globally certified and recommended by guidelines.
The world's first approved platelet-boosting drug for thrombocytopenia in chronic liver disease.
The world's first approved platelet-boosting drug for thrombocytopenia in chronic liver disease.
Rapid onset and potent efficacy
Takes effect in 3-5 days, reaches target platelet count in 5 days, with a response rate of 72.7% on and after day 8.
Doubles the maximum platelet count from baseline after treatment.
Doubles the maximum platelet count from baseline after treatment.

Lower costs and higher efficiency
The only TPO-RA proven to significantly reduce bleeding risk compared to placebo.
39% lower daily treatment cost than the national negotiation reference product, saving medical insurance funds and reducing patient burden.
39% lower daily treatment cost than the national negotiation reference product, saving medical insurance funds and reducing patient burden.

The following product information is intended for use by healthcare professionals only.
The following product may not have been approved and/or licensed for marketing in all countries where this website is accessible.
The following product may not have been approved and/or licensed for marketing in all countries where this website is accessible.

Directions for Use Download
(for medical professionals only)
(for medical professionals only)
Adverse Event Reporting
Email: pv@eddingpharm.com
Hotline: 0512-67611023
Product information stated by NMPA in Approved Drug Catalog of China.
【Name】 | Generic Name:Lusutrombopag Tablets Brand Name:MULPLETA®(稳可达®) Chinese Pinyin:Luqubopa Pian |
【Ingredients】 | Active Ingredient: Vancomycin Excipients:Mannitol, hydrochloric acid, water for injection, nitrogen gas. |
【Appearance】 | Tablets: 3 mg lusutrombopag as a light red, round, film-coated tablet debossed with the Shionogi trademark ( ) above the identifier code “551” on one side and with a “3” on the other side. |
【Indications】 | MULPLETA is a thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. |
【Specification】 | 3 mg |
| For detailed product information, please download the full package insert (PDF). | |
Directions for Use Download
(for medical professionals only)
(for medical professionals only)
Adverse Event Reporting
Email: pv@eddingpharm.com
Hotline: 0512-67611023
Guideline Recommendations
Academic Publications
Lusutrombopag for thrombocytopenia in Chinese patients with chronic liver disease undergoing invasive procedures
Journal: Hepatology International
Year: 2022
Read More
Year: 2022