Entinostat Tablets


The following product may not have been approved and/or licensed for marketing in all countries where this website is accessible.
(for medical professionals only)
Email: pv@eddingpharm.com
Hotline: 0512-67611023
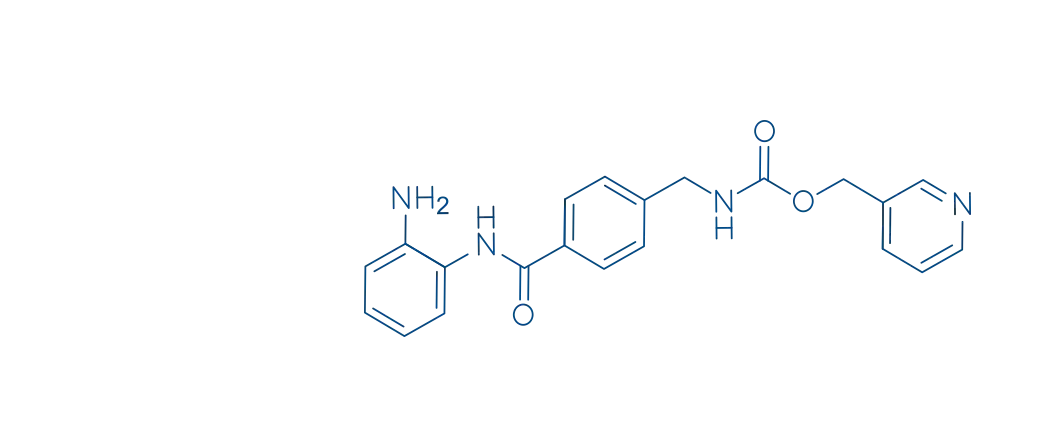
【Name】 | Generic Name:Entinostat Tablets Brand Name:景助达® Chinese Pinyin:Entisita Pian |
【Ingredients】 | Active Ingredient: Entinostat Excipients:Mannitol, Sodium Carboxymethyl Starch (CMS-Na), Hydroxypropyl Cellulose (HPC), Potassium Bicarbonate, Magnesium Stearate, Film-Coating Premix (Enteric-Soluble Type) [containing Hydroxypropyl Methylcellulose (HPMC), Talc, Titanium Dioxide, Red Iron Oxide/Yellow Iron Oxide, Polyethylene Glycol (PEG)]. |
【Appearance】 | Pink to light red (1mg) or yellow (5mg) film-coated tablet, which almost white after removing the coating. |
【Indications】 | in combination with an aromatase inhibitor for the treatment of women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer with disease progression following endocrine therapy. |
【Specification】 | (1)1mg;(2)5mg |
| For detailed product information, please download the full package insert (PDF). | |
(for medical professionals only)
Email: pv@eddingpharm.com
Hotline: 0512-67611023
Year: 2021
Entinostat, a class I selective histone deacetylase inhibitor, plus exemestane for Chinese patients with hormone receptor-positive advanced breast cancer: An overall survival update and long-term safety from the randomised, double-blind, placebo-controlled, phase 3 trial.
Year: 2024
Entinostat, a class I selective histone deacetylase inhibitor, plus exemestane for Chinese patients with hormone receptor-positive advanced breast cancer: A multicenter, randomized, double-blind, placebo-controlled, phase 3 trial
Year: 2023