Fluticasone Propionate Nebuliser Suspension
Half the dose of Fluticasone Propionate can improve nocturnal symptoms earlier.
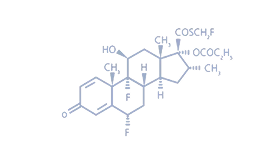
Half the dose of Fluticasone Propionate can significantly improve morning peak expiratory flow rate.

Fluticasone Propionate has less impact on children's growth and development; and has a lower incidence of oral candidiasis.

The following product may not have been approved and/or licensed for marketing in all countries where this website is accessible.

(for medical professionals only)
Email: pv@eddingpharm.com
Hotline: 0512-67611023
【Drug Name】 | Generic Name: Fluticasone Propionate Nebuliser Suspension Brand Name: Yirui Ping®(亿瑞平®) Chinese Pinyin: Bingsuan Futikasong Wuhua Xiruyong Hunxuanye |
【Ingredients】 | Active Ingredient: Fluticasone Propionate Excipients:Polysorbate20, Lauryl Sulfate, Disodium Hydrogen Phosphate Dihydrate, Disodium Phosphate Anhydrous, Sodium Chloride, Water for Injection. |
【Description】 | After shaking, this product becomes a white, opaque suspension. |
【Indications】 | Treatment of acute exacerbations of mild to moderate asthma in children and adolescents aged 4 to 16 years. Fluticasone propionate exerts a potent anti-inflammatory effect in the lungs. In asthma patients who have previously only received bronchodilators or other preventive treatments, Fluticasone Propionate can reduce asthma symptoms and acute exacerbations. While acute exacerbation symptoms can generally be relieved by using fast-acting bronchodilators, longer-lasting acute exacerbations require prompt treatment with glucocorticoids to control inflammation. |
【Specifications】 | 2ml:0.5mg |
| For detailed product information, please download the full package insert (PDF). | |
(for medical professionals only)
Email: pv@eddingpharm.com
Hotline: 0512-67611023
Zhang H, Shang YX, Shen KL, et al. Clinical study on the efficacy and safety of fluticasone propionate inhalation and oral prednisone in the acute exacerbation period of childhood asthma [J]. Chinese Journal of Practical Pediatrics, 2017, 32(9): 708-712.
Year: 2017
Cheng Q, Zhang H, Shang YX, et al. A multicenter clinical study on the efficacy and safety of fluticasone propionate suspension for nebulization in children aged 2-4 years with acute exacerbation of asthma [J]. Chinese Journal of Practical Pediatrics, 2023, 38(11): 846-852.
Year: 2023
Manjra AI, Price J, Lenney W, Hughes S, Barnacle H. Efficacy of nebulized fluticasone propionate compared with oral prednisolone in children with an acute exacerbation of asthma. Respir Med.2000;94(12):1206-1214.
Year: 2000