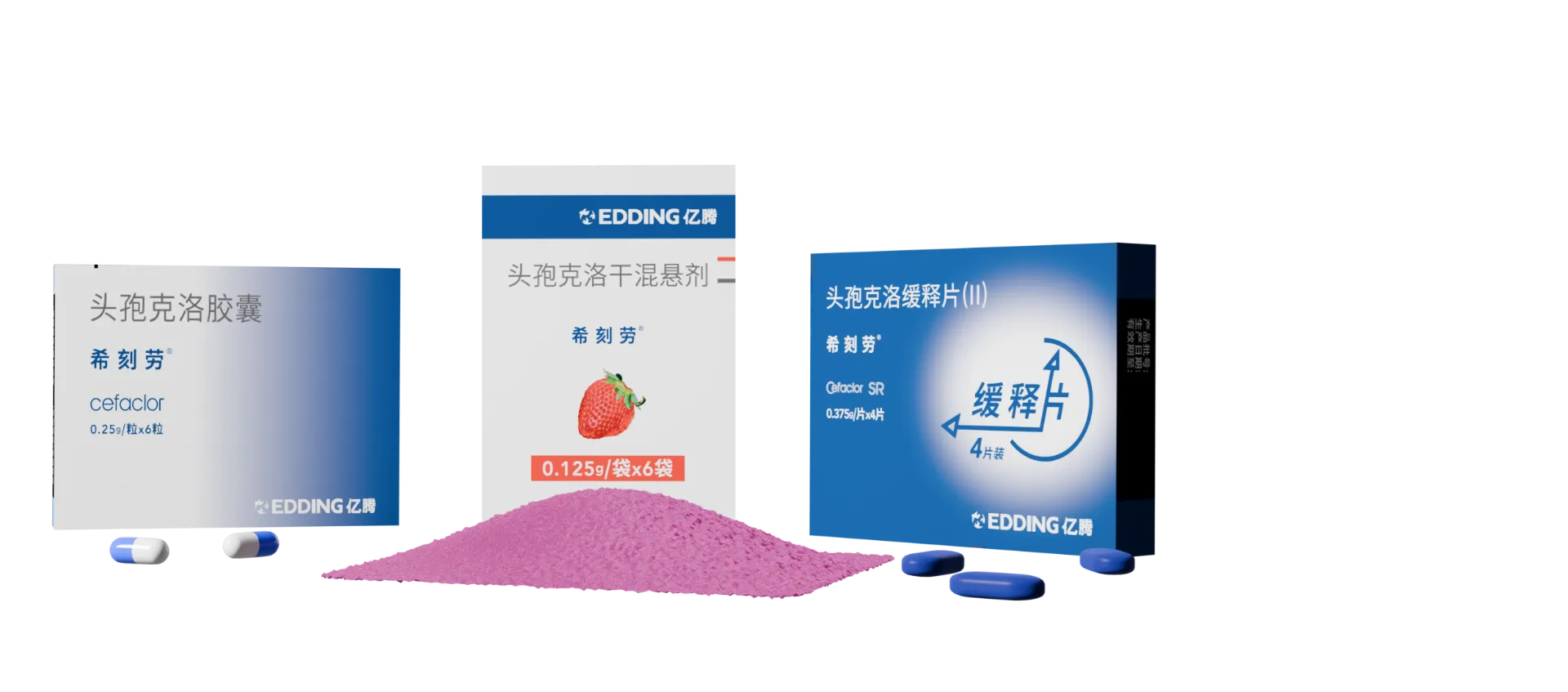
Cefaclor Suspension/Cer Capsules/
Cefaclor Extended-Release Tablets


The following product may not have been approved and/or licensed for marketing in all countries where this website is accessible.
(for medical professionals only)
Email: pv@eddingpharm.com
Hotline: 0512-67611023
【Drug Name】 | Generic Name: Cefaclor for Suspension Brand Name: Ceclor®(希刻劳®) Chinese Pinyin: Toubaokeluo Ganhunxuanji |
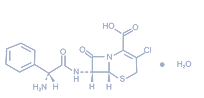
【Ingredients】 | Active Ingredient: Cefaclor |
【Appearance】 | Fine granules or powder; aromatic odor. |
【Indications】 | Cefaclor ® is indicated for the treatment of infections caused by susceptible strains of the following pathogens: Otitis media: caused by Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus species, Streptococcus pyogenes (Group A β-hemolytic streptococcus), and Moraxella catarrhalis. Lower respiratory tract infections (including pneumonia): caused by Streptococcus pneumoniae, Haemophilus influenzae, Streptococcus pyogenes (Group A β-hemolytic streptococcus), and Moraxella catarrhalis. Upper respiratory tract infections (including pharyngitis and tonsillitis): caused by Streptococcus pyogenes (Group A β-hemolytic streptococcus) and Moraxella catarrhalis. Note: Penicillin is commonly used to treat and prevent streptococcal infections (including prevention of rheumatic fever). The American Heart Association recommends amoxicillin for preventing bacterial endocarditis caused by dental, oral, and upper respiratory tract infections. In this context, penicillin V is a reasonable choice for preventing α-hemolytic streptococcal infections. Generally, Cefaclor is effective for eradicating nasopharyngeal streptococci. However, no significant data currently substantiates Cefaclor's efficacy for preventing secondary rheumatic fever or bacterial endocarditis. Treatment for β-hemolytic streptococcal infections should involve at least 10 days of Cefaclor therapy. Urinary tract infections (including pyelonephritis and cystitis): Caused by Escherichia coli, Proteus mirabilis, Klebsiella species, and coagulase-negative Staphylococcus. Note: Cefaclor is effective for both acute and chronic urinary tract infections. Skin and skin structure infections: Caused by Staphylococcus aureus and Streptococcus pyogenes (Group A β-hemolytic streptococci). Sinusitis Gonococcal urethritis Appropriate tissue culture and susceptibility testing should be performed to determine the sensitivity of the causative organism to Cefaclor. |
【Specifications】 | 0.125g(calculated as C15H14ClN3O4S) |
| For detailed product information, please download the full package insert (PDF). | |
【Drug Name】 | Generic Name: Cefaclor Sustained-Release Tablets (II) Brand Name:Ceclor®(希刻劳®) Chinese Pinyin: Toubao keluo Huanshipian(Ⅱ) |
【Ingredients】 | Active Ingredient: Cefaclor. |
【Appearance】 | Film-coated tablets; uncoated tablets appear off-white to pale yellow. |
【Indications】 | Cefaclor Sustained Release Tablets are indicated for the following infections caused by susceptible pathogens: Acute bronchitis and acute exacerbations of chronic bronchitis: Streptococcus pneumoniae, Haemophilus influenzae (including beta-lactamase-producing strain), Haemophilus parainfluenzae (including beta-lactamase-producing strains), Moraxella catarrhalis (including beta-lactamase-producing strains), and Staphylococcus aureus. Pharyngitis,tonsillitis: Caused by Streptococcus pyogenes (Group A streptococcus). (Penicillin is generally the drug of choice for treating and preventing streptococcal infections, including the prevention of rheumatic fever. Although Cefaclor Extended-Release Tablets are usually effective in clearing streptococci from the oropharynx, there is insufficient evidence to confirm that Cefaclor Extended-Release Tablets can prevent rheumatic fever attacks). Pneumonia: Caused by Streptococcus pneumoniae, Haemophilus influenzae (including beta-lactamase-producing strains), and Moraxella catarrhalis (including beta-lactamase-producing strains). Sinusitis: Caused by Streptococcus pneumoniae (penicillin-sensitive strains only), Haemophilus influenzae (including beta-lactamase-producing strains), and Moraxella catarrhalis (including beta-lactamase-producing strains). Uncomplicated lower urinary tract infections: Including cystitis and asymptomatic bacteriuria caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Staphylococcus saprophyticus. Skin and soft tissue infections: Caused by Streptococcus pyogenes (Group A streptococcus), Staphylococcus aureus (including beta-lactamase-producing strains), and Staphylococcus epidermidis (including beta-lactamase-producing strains). Bacteriological studies must be conducted to confirm the etiological diagnosis and determine the susceptibility of the pathogen to Cefaclor. Treatment should be initiated immediately after obtaining the appropriate specimens. Adjust the antimicrobial therapy based on the culture and susceptibility results. |
【Specifications】 | 0.375g(calculated as C15H14ClN3O4S) |
| For detailed product information, please download the full package insert (PDF). | |
【Drug Name】 | Generic Name: Cefaclor Capsules Brand Name:Ceclor®(希刻劳®) Chinese Pinyin: Toubaokeluo Jiaonang |
【Ingredients】 | Active Ingredient: Cefaclor. |
【Appearance】 | The contents are off-white to slightly yellowish powder or granules. |
【Indications】 | Cefaclor® is indicated for the following infections caused by susceptible pathogens: Acute bronchitis and acute exacerbations of chronic bronchitis: Streptococcus pneumoniae, Haemophilus influenzae (including beta-lactamase-producing strains), Haemophilus parainfluenzae (including beta-lactamase-producing strains), Moraxella catarrhalis (including beta-lactamase-producing strains), and Staphylococcus aureus. Pharyngitis, tonsillitis: Caused by Streptococcus pyogenes (Group A streptococcus). (Penicillin is generally the drug of choice for treating and preventing streptococcal infections, including rheumatic fever prophylaxis. Although Cefaclor Extended-Release Tablets are usually effective in clearing streptococci from the oropharynx, there is insufficient evidence to confirm that Cefaclor Extended-Release Tablets can prevent rheumatic fever attacks.) Pneumonia: Caused by Streptococcus pneumoniae, Haemophilus influenzae (including beta-lactamase-producing strains), and Moraxella catarrhalis (including beta-lactamase-producing strains). Sinusitis: Caused by Streptococcus pneumoniae (penicillin-sensitive strains only), Haemophilus influenzae (including beta-lactamase-producing strains), and Moraxella catarrhalis (including beta-lactamase-producing strains). Uncomplicated lower urinary tract infections: Including cystitis and asymptomatic bacteriuria caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Staphylococcus saprophyticus. Skin and soft tissue infections: Caused by Streptococcus pyogenes (Group A streptococcus), Staphylococcus aureus (including beta-lactamase-producing strains), and Staphylococcus epidermidis (including beta-lactamase-producing strains). Bacteriological studies must be conducted to confirm the etiological diagnosis and determine the susceptibility of the pathogen to Cefaclor. Treatment should be initiated immediately after obtaining the appropriate specimens. Adjust the antimicrobial therapy based on culture and susceptibility results. |
【Specifications】 | 0.375g(calculated as C15H14ClN3O4S) |
| For detailed product information, please download the full package insert (PDF). | |
(for medical professionals only)
Email: pv@eddingpharm.com
Hotline: 0512-67611023
Meyers BR. Cefaclor revisited. Clin Ther. 2000;22(2):154-166.
年份:2000