February 7, 2026 – EDG (06998.HK) is pleased to announce that its innovative small nucleic acid drug, EDP167, has successfully completed the first subject dosing in its Phase II clinical trial.
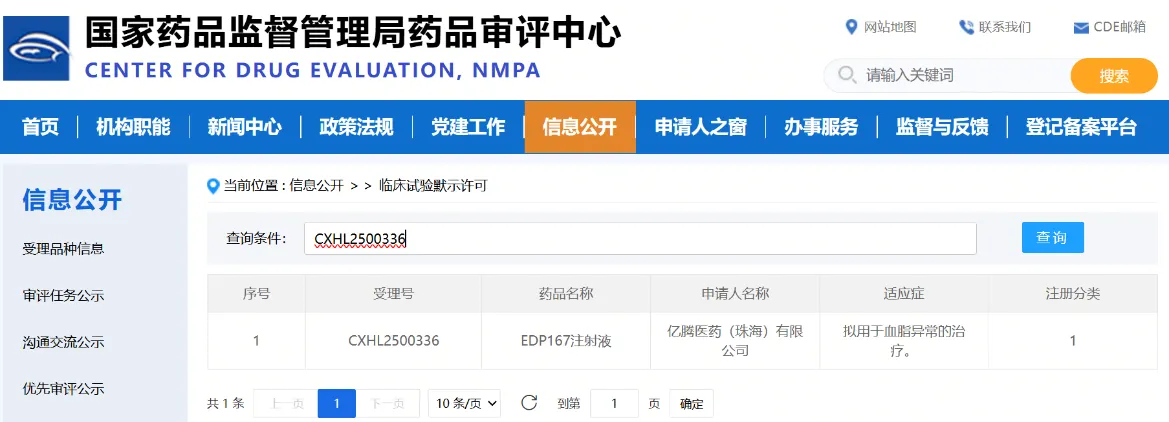
Phase II clinical trial of EDP167 is a multicenter, dose-finding and open-label trial in adult patients with Homozygous Familial Hypercholesterolemia (“HoFH”). The trial aims to evaluate the efficacy and safety of EDP167 in HoFH patients, with the primary endpoint being the change in low-density lipoprotein cholesterol (“LDL-C”) levels relative to baseline after 24 weeks of first dosing. Assessment of the primary endpoint is expected to be completed in the fourth quarter of 2026.
EDP167 is an innovative siRNA therapeutic designed for the treatment of dyslipidemia with unique clinical values.Prior to initiating Phase II trials in patients with HoFH, the Company completed a randomized, double-blind, placebo-controlled, single ascending dose Phase I study in healthy subjects and individuals with mild dyslipidemia. This study evaluated the safety, tolerability, pharmacokinetic, and pharmacodynamic characteristics of EDP167 across multiple dose levels. Results demonstrated that EDP167 exhibited favorable safety and tolerability profile. The data will be disclosed in the upcoming medical conference in 2026.
EDP167 is an innovative siRNA therapeutic designed for the treatment of dyslipidemia, developed in line with one of the Company’s strategic focuses on cardiovascular diseases. The drug targets hepatic angiotensin-like protein 3 (“ANGPTL3”)-a key regulator secreted by the liver that simultaneously inhibits lipoprotein lipase and endothelial lipase, thereby modulating the metabolism of multiple atherogenic lipoproteins. Genetic studies confirm that individuals with loss-of-function mutations in ANGPTL3 have a significantly reduced risk of atherosclerotic cardiovascular disease.
Through precise and sustained silencing of hepatic ANGPTL3 expression, EDP167 achieves a dual potent reduction in LDL-C and triglyceride (“TG”) levels. Importantly, its mechanism of action is independent of the LDL receptor (“LDLR”) pathway, thereby overcoming the limitations of conventional lipid-lowering agents, including statins and PCSK9 inhibitors, which rely on LDLR function. This unique feature of EDP167 provides an important innovative treatment option for patients with dyslipidemia, especially those with HoFH or mixed dyslipidemia, and offers broad clinical application prospects.
In recent years, EDG has rapidly expanded its R&D pipelines for bispecific/multispecific antibodies and small nucleic acid drugs, establishing three core technology platforms: the Bi/multi-specific Antibodies Technology Platform, the T-Cell Engagers (TCEs) Technology Platform, and the Small Nucleic Acid Technology Platform. For small nucleic acid drugs, in addition to EDP167, which has entered Phase II clinical development, several other products targeting hyperlipidemia, renal disease, and liver disease are in early-stage development. Furthermore, the Company is steadily advancing multiple clinical trials in the autoimmune and oncology sectors, with two TCE trispecific antibodies in its pipelines currently prepared for clinical trial applications.
As a biopharmaceutical enterprise with billions of yuan in annual sales revenue while maintaining a robust pipeline of First-in-Class (FIC) and BIC candidates, EDG continues to leverage its stable commercial income to fuel upstream R&D. This model accelerates the transition of drug candidates through every stage, from molecule discovery to clinical trials and regulatory approval. Currently, no domestically developed siRNA drug has been approved in China. We look forward to the continued progress of clinical research of EDP167, and hope it will stand out in a competitive landscape, fill the existing therapeutic gap, and provide a new treatment option for more cardiovascular patients.
ABOUT EDG
The Company is an integrated specialty biopharmaceutical company, focusing on oncology, autoimmune diseases, cardiovascular diseases, respiratory diseases, and anti-infectives. Through acquisition of branded drug assets from multinational pharmaceutical companies (“MNC”) and licensing in development and commercialisation rights of innovative patented drugs from global biopharmaceutical companies, the Company has established a competitive portfolio of originatorbranded drugs and innovative drugs. The Company has successfully brought multiple innovative drugs to market in China, reflecting its strong clinical development and management capabilities.Moreover, the Company has demonstrated high-quality manufacturing, supply chain management, technology transfer and quality control systems through operating the production facilities and management systems transferred from MNCs in its historical asset acquisitions.For more information, please visit www.eddingpharm.com.